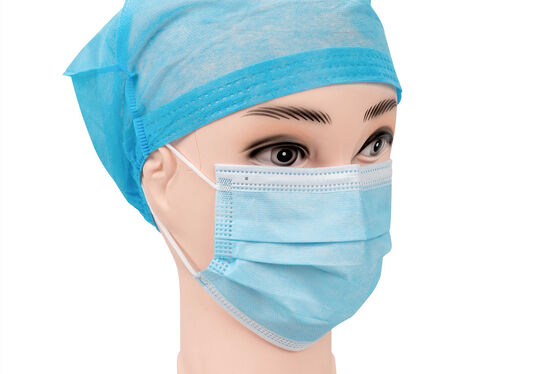
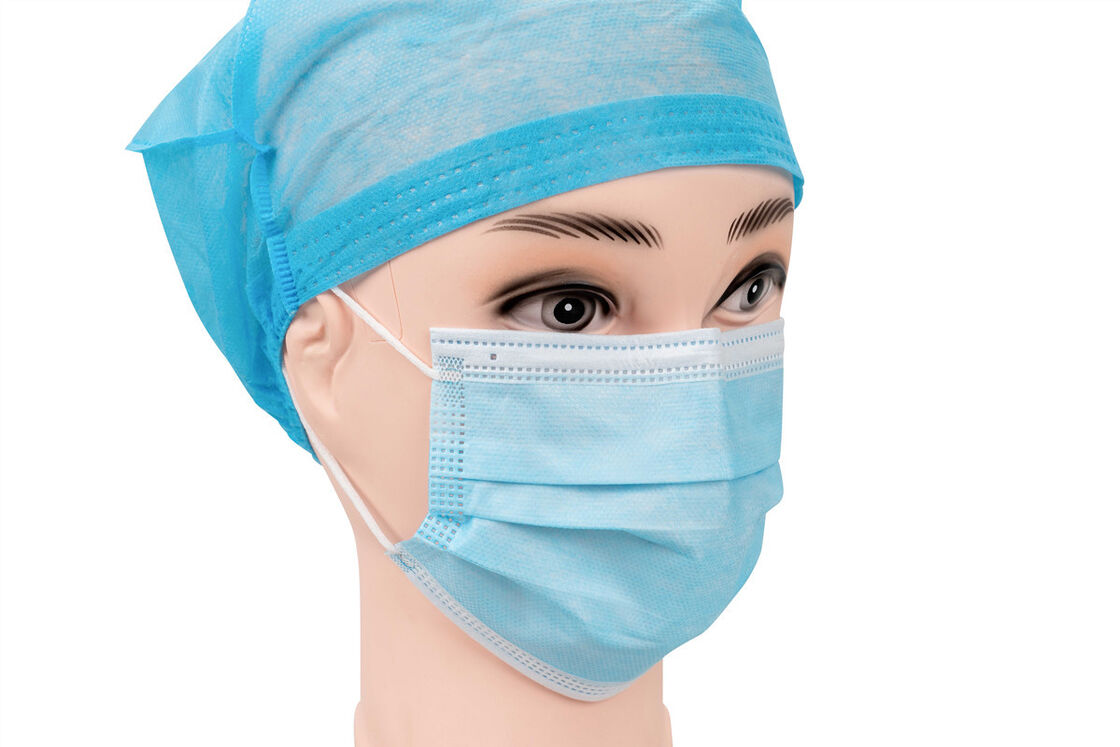
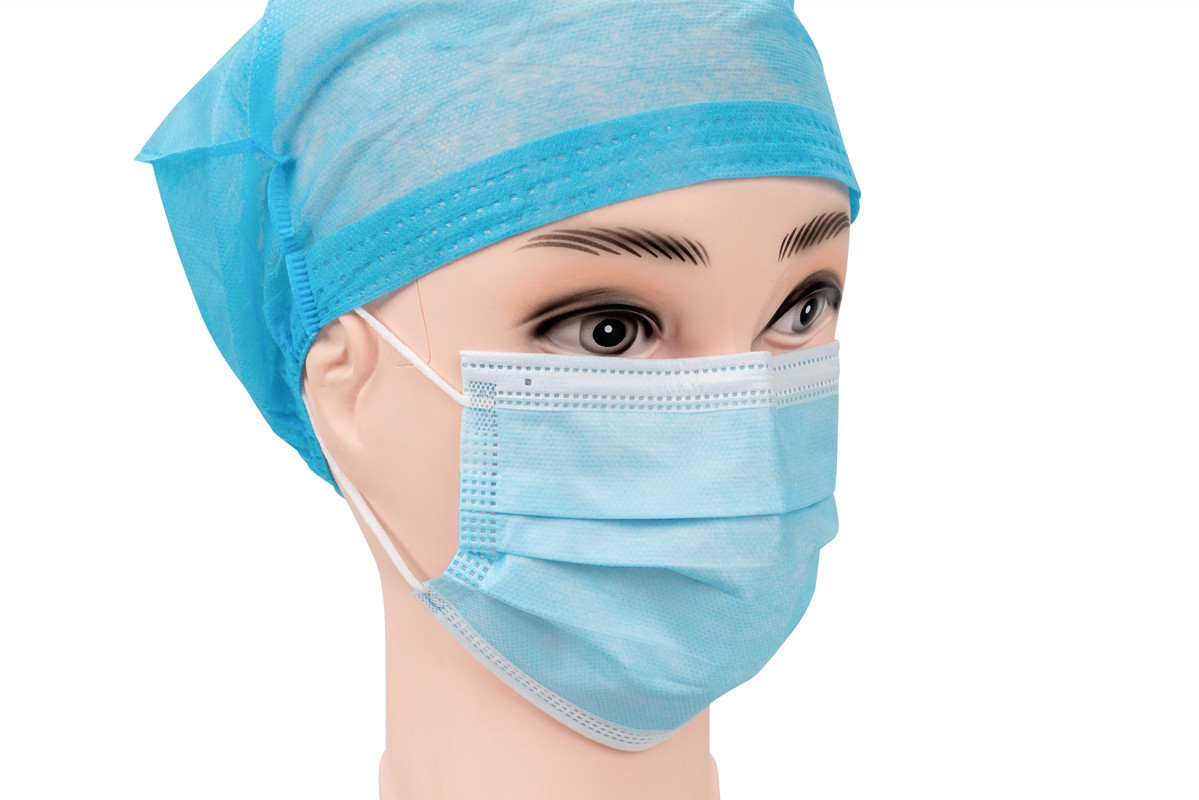
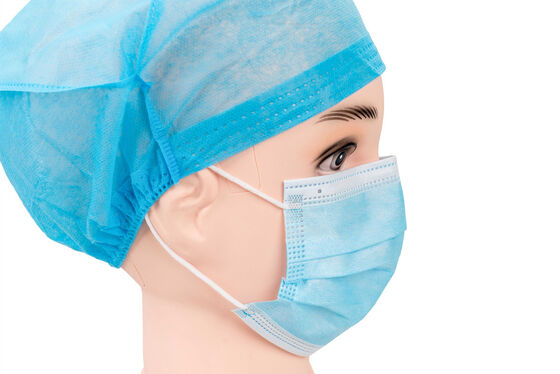
Product Description
EN14683 Type-IIR Medical Face Mask
EN 14683 is the main European standard that specifies the requirements and test methods for medical face masks. These are often called "surgical masks" or "procedure masks." The standard classifies masks into different types based on their Bacterial Filtration Efficiency (BFE) and other key properties. And Type IIR is the highest performing level within this standard for liquid splash resistance.
Key Requirements for Type IIR Masks:
- Bacterial Filtration Efficiency (BFE): ≥ 98% (Very high protection against bacteria in aerosols).
- Breathability (Delta P): < 60 Pa/cm². While still comfortable, it may feel less breathable than lower-type masks.
- Splash (Synthetic Blood): Must withstand a pressurized jet of synthetic blood without penetration. The "R" stands for "Resistant" to splashes.
- Microbial Cleanliness: Has low levels of microbial contamination on the mask itself.
Product Informations:
| Material |
polypropylene, meltblown |
| Layer |
3ply |
| Wearing |
earloop |
| Size |
17.5x9.5cm |
| Color |
white, green, blue, black |
| BFE |
>98% |
| PFE |
>98% |
| Use For |
operating rooms, surgical procedures, emergency rooms, etc |


About Us:
Hubei TDL Group is made of Hubei Orient International Trading Corporation and Ammex-Weida(Hubei) Health and Safety products Co.,Ltd. We specilize in the design, manufacturing and sales of disposable medical products and PPE products.
Hubei Orient owns the factory: Ammex-Weida(Hubei) Health and Safety Products Co. Ammex-Weida is situated in Xiantao, Hubei, China. Ammex-weida established in 2003, covers 65,000 square meters. We are a group of more than 500 individuals, and equipped with a medical and industrial laboratory of Class 100,000 (ISO 8 equivalent) and clean-room workshops of Class 10,000 (ISO 7 equivalent) for the production of sterile medical devices and various disposable products. Our disposable products are exported to more than 60 countries and regions in Europe, America, Asia, Middle East etc.

Our group has more than 20 individuals for QC quality control, laboratory and inspection. Our laboratory could do tests for face masks such as particulate filtration efficiency, differential pressure, synthetic blood penetration, tensile test for earloop, flammability test, microbial cleanliness, splash resistance, sterile test, ethylene oxide residue etc. And we could do tests for coveralls such as hydro-static pressure, repellency by liquids, resistance to penetration by liquids, surface resistance, resistance to Ignition, filtering efficiency, puncture resistance, tensile strength, static decay period, sterile test, ethylene oxide residue etc. And also we could do tests for surgical gowns such as resistance of microbial penetration, cleanliness microbial, particle release, liquid penetration, bursting strength, tensile strength.

Cleanroom face mask workshop in class 100000: all kinds of disposable medical facemask, KN95 folded masks, 3D masks, cleanroom masks, KF94 masks. The daily output is around 5 million pieces totally.


We utilize a complete quality management system ISO9001, ISO13485. We care about human rights and we are certified by human right agency of SEDEX. Ammex-Weida has medical device license for class I and II. Our products are certified with CE,FDA. Our medical face masks passed through Nelson's test. Our medical face masks are conform to EN14683 type II and type IIR for Europe market, ASTM level-3 510K for North America and the national standard YY0469 and YY0969 for China market. Our category III protective coveralls are complied with new European PPE Regulations (EU)2016/425. They are classified to type 3/4/5/6 and 3B/4B/5B/6B. And we also have certified with EcoVadis and ISO14001.


 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!